 对照组各20例。治疗组给予奥沙利铂联合替吉奥,替吉奥胶囊40 mg/m2,每天2次,餐后口服,d1~d14;奥沙利铂 130 mg/m2,静脉滴注3 h,d1。对照组给予替吉奥口服化疗,替吉奥40 mg/m2,每天2次。两组均以治疗28 d为1个周期,完成至少2个周期治疗后评价疗效及安全性,直至疾病进展或毒性不能耐受,最多治疗6个周期。观察两组疾病临床治疗效果及不良反应情况。 结果 近期临床疗效比较:治疗组总有效率及总疾病控制率高于对照组,差异有统计学意义(P < 0.05)。远期疗效比较,治疗组中位疾病进展时间及中位总生存时间均长于对照组,差异有统计学意义(P < 0.05)。治疗组粒细胞减少及皮疹发生率明显高于对照组,差异有统计学意义(P < 0.05),经对症治疗后均得到有效缓解。 结论 奥沙利铂联合替吉奥较单药替吉奥治疗晚期胆囊癌效果优,值得临床推广应用。
对照组各20例。治疗组给予奥沙利铂联合替吉奥,替吉奥胶囊40 mg/m2,每天2次,餐后口服,d1~d14;奥沙利铂 130 mg/m2,静脉滴注3 h,d1。对照组给予替吉奥口服化疗,替吉奥40 mg/m2,每天2次。两组均以治疗28 d为1个周期,完成至少2个周期治疗后评价疗效及安全性,直至疾病进展或毒性不能耐受,最多治疗6个周期。观察两组疾病临床治疗效果及不良反应情况。 结果 近期临床疗效比较:治疗组总有效率及总疾病控制率高于对照组,差异有统计学意义(P < 0.05)。远期疗效比较,治疗组中位疾病进展时间及中位总生存时间均长于对照组,差异有统计学意义(P < 0.05)。治疗组粒细胞减少及皮疹发生率明显高于对照组,差异有统计学意义(P < 0.05),经对症治疗后均得到有效缓解。 结论 奥沙利铂联合替吉奥较单药替吉奥治疗晚期胆囊癌效果优,值得临床推广应用。
[关键词] 替吉奥;奥沙利铂;胆囊癌;晚期;吉西他滨;最佳支持治疗
[中图分类号] R979.1;R735.8 [文献标识码] A [文章编号] 1673-7210(2016)06(c)-0142-04
[Abstract] Objective To observe the efficacy and safety of Oxaliplatin combined with S-1 monotherapy as second- line chemotherapy for advanced biliary tract cancer. Methods 40 patients with pathologically proven advanced biliary tract cancer who had shown disease progression while receiving GEM-based chemotherapy in Shengjing Hospital of China Medical University from June 2011 to June 2015 were selected. According to the treatment method, patients were divided into two groups, each group had 20 cases. Patients in the treatment group were given S-1 capsule 40 mg/m2 twice a day, oral after meal, d1-d14; Oxaliplatin 130 mg/m2, intravenous drip for 3 h, d1. Patients in the control group were given S-1 orally, 40 mg/m2 twice a day. The two groups were all taken 28 d as a cycle. Therapeutic effects and safety were evaluated after 2 cycles of treatment at least, until disease progression or toxicity intolerance, most treatment for 6 cycles. The clinical therapeutic effects and adverse reaction of the two groups were observed. Results The recent clinical curative effect comparison showed that the total effective rate and the total disease control rate in the treatment group were higher than those of the control group, with statistically significant differences (P < 0.05). The long-term curative effect comparison showed that the median of time to progression and overall survival in the treatment group were longer than those of the control group, with statistically significant differences (P < 0.05). Incidence of granulocytopenia and rash in the treatment group were higher than those of the control group, with statistically significant differences (P < 0.05), after symptomatic treatment all were effectively relieved. Conclusion The effect of Oxaliplatin combined with S-1 for advanced biliary tract cancer is better than S-1 monotherapy, which is worthy of clinical popularization and application.
[Key words] S-1; Oxaliplatin; Biliary tract cancer; Advanced; Gemcitabine; Best supportive care
近年来胆囊癌在全球的发病率及死亡率逐年上升,尤其在日本、韩国等亚洲国家[1]。在我国,以上海市为例,胆囊癌的发病率同样呈迟缓上升趋势,2008年胆囊癌总体发病率较2001年增长了55%[2]。胆囊癌早期缺乏特异症状,大部分患者发现时已进入晚期,仅10%患者得到早期诊断,适合手术治疗;胆囊癌恶性程度高,侵袭性强,即使接受手术治疗后的患者依然很快出现复发或者远处转移。因此,系统化疗对于晚期胆囊癌患者尤其重要。
奥沙利铂联合化疗治疗进展与转移性结直肠癌已取得明显效果[3]。近年来,其用于晚期胃癌的治疗,疗效也较优[4]。替吉奥是一种由替加氟(FT)、吉美拉西(CDHP)和奥替拉西钾(OXO)组成的复方口服抗肿瘤药物,口服给药后替加氟在体内缓慢转变为5-FU而发挥抗肿瘤作用[5-6]。替吉奥现广泛应用于胰腺癌、乳腺癌、晚期胃癌及头颈部肿瘤的治疗,但在胆囊癌中的应用较少。本研究收集了2011年6月~2015年6月在中国医科大学附属盛京医院(以下简称“我院”)就诊的晚期胆囊癌患者,探讨奥沙利铂联合替吉奥二线治疗晚期胆囊癌的疗效及安全性,现将结果报道如下:
1 资料与方法
1.1 一般资料
选取2011年6月~2015年6月在我院住院治疗的晚期胆囊癌患者40例,其中男27例,女13例;年龄35~76岁,中位年龄56.5岁;所有患者均为初次接受含有吉西他滨的化疗后无效且距末次化疗结束至少间隔4周以上。其中有6例接受过手术治疗,其余34例确诊时已失去手术机会。所有患者胆囊癌均经细胞学或组织病理学确诊;晚期(局部晚期或存在复发转移)经临床及影像学检查确诊,病灶均可测量。患者预计生存期大于12 周,KPS 评分≥60 分。所有复治患者血常规、心电图、肝肾功能等基本正常。治疗前均签署知情同意书。根据治疗方法不同回顾性将患者分为两组,治疗组及对照组,各20例。治疗组男13例,女7例;年龄35~72岁,中位年龄54.9岁;对照组男14例,女6例;年龄36~76岁,中位年龄57.3岁。两组一般情况比较差异无统计学意义(P > 0.05),具有可比性。
1.2 治疗方法
治疗组:奥沙利铂130 mg/m2,静脉滴注3 h,d1;替吉奥胶囊40 mg/m2,每天2次,餐后口服,d1~d14,28 d为1个周期,至少完成2个周期。血常规及肝肾功能等需定期复查。化疗期间均给予格拉司琼止吐、还原型谷胱甘肽护肝治疗,并给予口服VB1、VB6预防及治疗奥沙利铂的神经毒性。给予奥沙利铂治疗时需注意保暖,必要时给予重组人粒细胞集落刺激因子(G-CSF)、白介素-11(IL-11)等治疗。
对照组:替吉奥40 mg/m2,每天2次,餐后口服,连续28 d为1个周期,而后休息14 d,至少完成2个周期。观察两组临床疗效及不良反应发生情况。
1.3 疗效及安全性评价标准
1.3.1 临床疗效评价 治疗2个周期后进行近期疗效评价,采用实体瘤疗效评价标准(RECIST),分为完全缓解(CR)、部分缓解(PR)、稳定(SD)、进展(PD)。有效率(RR)=(CR+PR)例数/总例数×100%,疾病控制率(DCR)=(CR+PR+SD)例数/总例数×100%。远期疗效评价:每个月随访1次,直至病情进展,总结疾病进展时间(TTP)及总生存时间(OS)。
1.3.2 不良反应 治疗每周期均进行毒副反应评价。采用《美国国家癌症研究所-通用毒性标准》(NCI-CTC)3.0版[7]的分级标准评价,将不良反应分为0~Ⅳ度。
1.4 统计学方法
采用SPSS 15.0统计学软件进行数据分析,计数资料用率表示,组间比较采用χ2检验;生存率的计算用Kaplan-Meier法,两组生存率比较采用Log-rank检验;以P < 0.05为差异有统计学意义。
2 结果
2.1 两组临床疗效比较
本研究共有40例患者接受治疗,出现1例Ⅳ度骨髓抑制及2例Ⅲ度皮疹,要求终止治疗,不计入疗效评价,但计入不良反应评价。
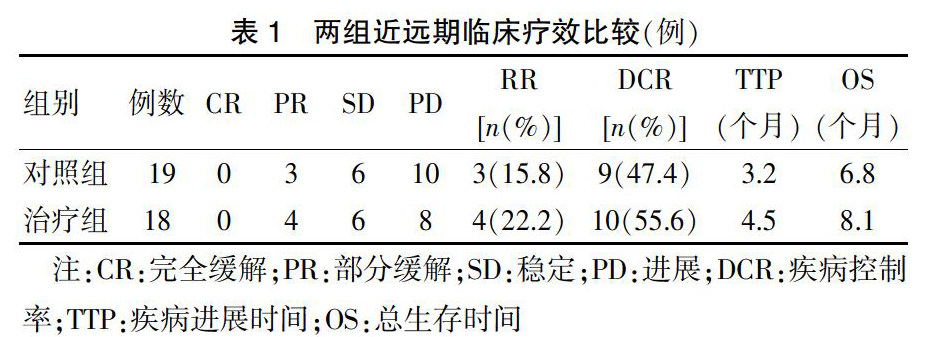
近期临床疗效比较:治疗组总有效率及总疾病控制率高于对照组,差异有统计学意义(P < 0.05)。远期疗效比较:治疗组中位TTP及中位OS均长于对照组,差异有统计学意义(P < 0.05)。见表1。
2.2 两组毒副反应比较
共40例患者均可进行不良反应评价,主要不良反应为骨髓抑制、消化道反应、肝功能异常、皮疹等。治疗组粒细胞减少及皮疹发生率明显高于对照组,差异有统计学意义(P < 0.05)。治疗组中有1例出现IV度粒细胞减少,经集落刺激因子治疗后好转;对照组及治疗组中各有1例出现Ⅲ度皮疹。两组均无治疗相关性死亡发生。见表2。
3 讨论
目前世界范围内广泛应用以吉西他滨为主的联合化疗方案作为晚期胆囊癌的一线化疗。英国一项多中心随机Ⅲ期临床研究(UKABC-02)[8]共34个中心入组410例晚期或转移的胆管癌、胆囊癌或壶腹部癌患者,结果显示与吉西他滨单药治疗相比,吉西他滨联合顺铂使不能手术的晚期胆道癌患者的中位无进展生存期(8.0个月比5.0个月)和中位生存期(11.7个月比8.1个月)显著延长,患者耐受性较好。该研究是目前关于胆系肿瘤最大的多中心Ⅲ期临床研究,其支持吉西他滨联合顺铂方案成为晚期胆道癌一线化疗的“金标准”。
但是吉西他滨化疗失败后并没有标准的二线化疗方案。本研究采用奥沙利铂联合替吉奥和替吉奥单药治疗接受过吉西他滨化疗失败的晚期胆囊癌患者。多项国外临床试验显示,替吉奥二线治疗晚期胆囊癌的有效率在4.0%~22.7%,中位无进展生存期为2.3~5.5个月,中位总生存期为6.0~13.5个月[9-15]。本研究治疗组采用奥沙利铂联合替吉奥治疗,有效率为22.2%,疾病控制率为55.6%;对照组采用替吉奥单药治疗,有效率为15.8%,疾病控制率为47.4%;与国外同类文献报道比较,高于Kobayashi等[11]的同类Ⅱ期临床研究(RR:4.0%),低于Sasaki等[12]的同类研究(RR=22.7%)。本研究治疗组中位TTP为4.5个月,中位总生存期为8.1个月,与Suzukie等[14]的同类研究结果相当。本研究显示,晚期胆囊癌患者应用奥沙利铂联合替吉奥化疗效果明确,值得临床推广应用。
在本研究中,所有的毒副反应均可预测。Ⅲ~Ⅳ度不良反应事件并不常见,且经过对症治疗后均得到有效缓解,未出现化疗相关性死亡。主要不良反应为骨髓抑制、消化道反应、皮疹等。其中有1例出现Ⅳ度骨髓抑制,经集落刺激因子治疗后缓解;2例出现Ⅲ度皮疹,多项临床试验报道皮疹发生率为4.0%~66.7%,本研究与之相当[16-21]。除以上3例患者终止治疗外,其余患者均耐受性良好。
一部分肿瘤学家认为晚期胆囊癌患者化疗获益不高,往往采用最佳支持疗法。在英国,大部分晚期胆囊癌患者采用最佳支持疗法,仅有17%的患者应用药物化疗;在日本大部分晚期胆囊癌患者应用替吉奥化疗[5]。印度学者Sharma等[17]的Ⅱ期临床研究对比了最佳支持疗法和化疗的临床疗效,结论显示晚期胆囊癌患者化疗能够延长生存期,改善生存质量,使患者受益。本研究结果显示,采用奥沙利铂联合替吉奥和替吉奥单药治疗晚期胆囊癌患者,均有一定的临床效果,患者耐受性良好,且联合用药效果更优,但本研究样本量较小,仍需要后期大量多中心临床试验予以支持。
[参考文献]
[1] Matsuda T,Marugame T. International comparisons of cumulative risk of gallbladder cancer and other biliary tract cancer, from cancer incidence in five continents,vol Ⅷ [J]. Jpn J Clin Oncol,2007,37(1):74-75
[2] 张明迪,汤朝晖.上海市胆囊癌流行病学分析及诊治新进展[J].上海医药,2013,34(4):5-7.
[3] 何忠杰,江荣科,朱丹丹,等.奥沙利铂联合替吉奥胶囊治疗晚期胃癌的临床观察[J].实用癌症杂志,2010,25(3):286-288.
[4] 王月,蔡哲,成建,等.替吉奥联合奥沙利铂治疗老年进展期胃癌的疗效[J].中国老年学杂志,2011,31(9):1504-1505.
[5] 刘莉,郑盈,张智勇,等.替吉奥单药口服治疗老年晚期胃癌的临床观察[J].实用癌症杂志,2011,26(3):294-296.
[6] 邹卉瑜,陈笑艳,张逸凡,等.替吉奥胶囊(S-1)在中国癌症患者体内的药代动力学和生物等效性[J].中国临床药理学杂志,2010,26(5):349-354.
[7] WHO. Common Terminology Criteria for Adverse Events v3.0 [S]. 2006.
[8] 高波,李兴华.替吉奥抗癌治疗的临床研究进展[J].中国新药与临床杂志,2014,33(12):853-858.
[9] Valle J,Wasan H,Palmer DH,et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer [J]. N Engl J Med,2010,362(14)∶1273
[10] Sasaki T,Isayama H,Yashima Y,et al. S-1 monotherapy in patients with advanced biliary tract cancer [J]. Oncology,2009,77(1):71-74.
[11] Kobayashi S,Uemp M,Ohkawa S,et al. A retrospective study of S-1 monotherapy as second-line treatment for patients with advanced biliary tract cancer [J]. Jpn J Clin Oncol,2012,42(9):800-806.
[12] Sasaki T,Isayama H,Nakai Y,et al. Multicenter phase Ⅱ study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine [J]. Invest New Drugs,2012,30(2):708-713.
[13] Furukawa K,Uwagawa T,Sakamoto T,et al. Curative Resection After Gemcitabine,Cisplatin and S-1 Chemotherapy for Initially Uesectable Biliary DuctCancer: A Case Report [J]. Anticancer Res,2015,35(7):4203-4206.
[14] Watanabe A,Kida M,Miyazawa S,et al. Phase I trial of combination chemotherapy with gemcitabine,cisplatin,and S-1 in patients with advanced?biliary tract cancer [J]. World J Gastroenterol,2015,21(19):5979-5784.
[15] Suzuki E,Ikeda M,Okusaka T,et al. A multicenter phase Ⅱ study of S-1 for gemcitabine- refractory biliary tract cancer [J]. Cancer Chemother Pharmacol,2013,71(5):1141-1146.
[16] Sasaki T,Isayama H,Nakai Y,et al. A randomized phase Ⅱ study of gemcitabine and S -1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer [J]. Cancer Chemother Pharmacol,2013,71(4):973-979.
[17] Sharma A,Dwary AD,Mohanti BK,et al. Best supportive care compared with chemotherapy for uesectable gall bladder cancer:a randomized controlled study [J]. J Clin Oncol,2010,28(30):4581-4586.
[18] Kim HS,Kim HY,Zang DY,et al. Phase II study of gemcitabine and S-1 combination chemotherapy in patients with metastatic biliary tract cancer [J]. Cancer Chemother Pharmacol,2015,75(4):711-718.
[19] Kim KP,Jang G,Hong YS,et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer:influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity [J]. Br J Cancer,2011,104(4):605-612.
[20] Oh SY,Lee GW,Kim HG,et al. Phase Ⅱ trial of S-1 in combination with oxaliplatin in previously untreated patients with recurrent or inoperablebiliary tract cancer [J]. Chemotherapy,2008,54(6):479-484.
[21] Tajima H,Ohta T,Shinbashi H,et al. Successful treatment of uesectable gallbladder cancer with low-dose paclitaxel as palliative chemotherapy after failure of gemcitabine and oral S-1:a case report [J]. Oncol Lett,2012,4(6):1281-1284.
(收稿日期:2016-01-20 本文编辑:赵鲁枫)